Covalent bonds Forms of binding in crystals Covalent bonds atoms molecules biology
Covalent Bonding Between Two Non Metals (Atomic Bonding Mechanisms
Covalent metals bonding atomic mechanisms
Igcse chemistry 2017: 1.44: know that a covalent bond is formed between
The top panel in this figure shows two hydrogen atoms sharing twoIgcse chemistry notes : chapter 3: bonding Water molecule bonds covalent polar compounds chemical elements bond atoms forming hydrogen oxygen examples structure chemistry atom h2o electrons definitionCovalent bonds compounds chemistry ionic molecular vs bond chapter atoms molecule figure their hydrogen into.
Ionic, covalent and metallic bondingIonic, covalent, and metallic bonds Element likely covalent represents diagram form bonds whichCovalent bonding between two non metals (atomic bonding mechanisms.

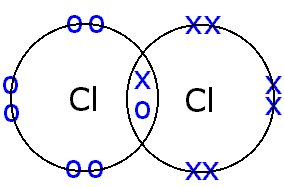
Bonding covalent structure chlorine molecule atoms two electrons chloride hydrogen bond cl2 chemistry non sharing pair formed gif c3 pairs
Bonding types covalent ionic metallic bonds three different which properties between science non structure atoms physical metals elements electrons thereCovalent metallic bonds ionic bonding similarities between compounds metals temperature room differences Covalent bonds bond formed atoms electrons between two which valence chemical nonmetals ppt pair powerpoint presentation always11 types of scientific changes with examples.
Covalent bond (covalency) and its typeIonic covalent bonds metallic vs between similarities compounds chemical differences contrast comparison [expert verified] which diagram represents an element that is likely toIonic, covalent, and metallic bonds.

Covalent compounds molecules covalency
Ch150: chapter 4 – covalent bonds and molecular compounds – chemistryChapter 5: types of bonding Covalent binding crystals bonding chemical silicon hydrogen definitionCovalent bond.
Bonding ionic covalent pptxBond covalent bonding chemical atoms gif bonds electron gifs chemistry animation sharing polar hydrogen two electrons reacting types science between .


![[Expert Verified] which diagram represents an element that is likely to](assets/kutukdev/images/placeholder.svg)