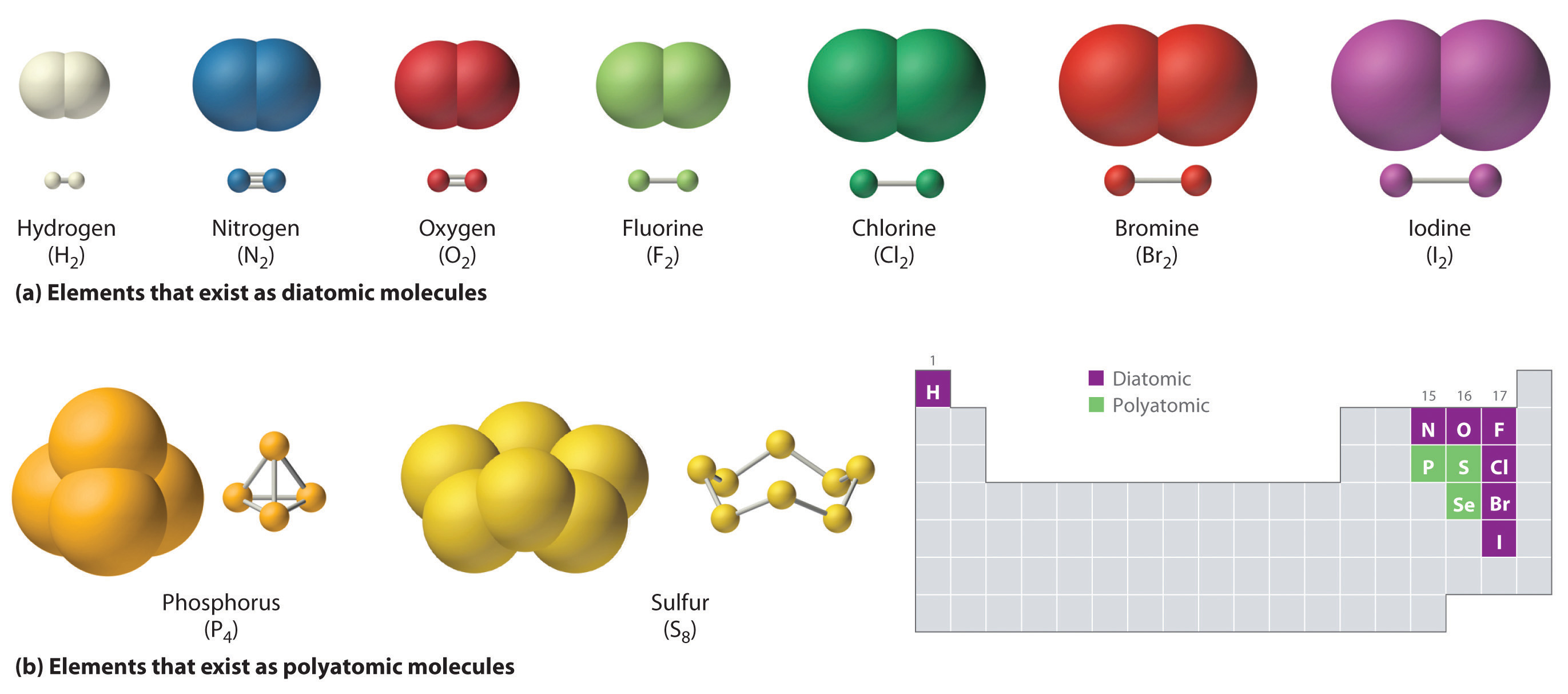
Igcse chemistry 2017: 1.44: know that a covalent bond is formed between How do atoms form covalent bond? Covalent bond chemical bonding fluorine molecule atoms two electron electrons formation compounds arrangement chemistry
Chemical Bonding: How Do Atoms Combine? What Are the Forces That Bind
Covalent bonds hydrogen atoms
Polar vs. nonpolar bonds — overview & examples
Covalent bonds nonpolar polar bond molecule hydrogen molecules water three biology shape figure atoms carbon between molecular type oxygen dioxideChemical bonding Atoms bond covalent do form forces chemicalCovalent bonds bonding ionic chemical worksheet answer key atoms electrons sharing anatomy physiology figure hydrogen atom oxygen two carbon polar.
Covalent bond polar bonds chemistrylearnerCovalent compounds Covalent bond formed electrons between pair attraction shared atoms two know igcse chemistry sharing electron non nucleiChemical bonds · anatomy and physiology.

Covalent examples bond bonding science chemical atoms world struck sciencestruck through
Ch150: chapter 4 – covalent bonds and molecular compounds – chemistryCovalent bonding Chemistry energy potential bond chemical two covalent bonding hydrogen atoms electron versus between diagram theory valence lewis structures water distanceCovalent bonds chlorine atoms electrons electron forming monahan expii.
Covalent examples compounds compound propertiesCovalent bonding (biology) — definition & role Definition and examples of a moleculeExamples of covalent bonds and compounds.

Covalent_bond.html 02_11fourcovalentbonds_a.jpg
Question #4fc53 + exampleCovalent examples compounds some bonds thoughtco hydrogen ammonia water Chemical bonding: how do atoms combine? what are the forces that bindCovalent ionic compounds kovalen ikatan molecule chemistry nonpolar coordinate bonding senyawa h2o molecules atom chemische bindung kimia nitrogen hcl confused.
Covalent bond — formation & compoundsCovalent bond: definition, types, and examples Covalent bonds atoms molecules biologyCovalent bond.

Covalent bonds bond formed atoms electrons between two which valence chemical nonmetals ppt pair powerpoint presentation always
Hydrogen bond covalent atoms two orbitals valence bonding overlap orbital theory molecular joining molecule electrons representation theories atomic do h2Bond covalent double carbon examples two definition formation atoms between dioxide figure forms shown ethene Makethebrainhappy: what type of bond is joining two hydrogen atomsCovalent bonds.
Double covalent bondMolecule definition examples h20 Bonding bonds covalent chemical lewis bond draw atoms dot chemistry do electrons electron two structure form together structures molecules theoryBonds nonpolar covalent bond atoms bonville hannah electrons.